Health Ministry answers frequently asked questions about COVID-19 vaccine as more than 1 mln shots given
Turkey's Health Ministry has answered the most frequently asked questions on the COVID-19 vaccine on its online information platform, as the number of people who have received their first dose of Chinese producer Sinovac's COVID-19 vaccine exceeded 1 million as of Jan. 20 in the country. Meanwhile, journalist Fatih Altaylı said that Turkey plans to purchase the COVID-19 vaccine developed by AstraZeneca and Oxford University through Russia.
Duvar English
Turkey has vaccinated more than 1 million people in the first week of its nationwide rollout of COVID-19 shots developed by China's Sinovac Biotech Ltd, Health Ministry data showed on Jan. 20.
The program was launched on Jan. 14, starting with health workers and then including the elderly. More than 600,000 people were vaccinated in the first two days but the pace has slowed since then to around 100,000 people per day.
The Health Ministry on its online information platform answered the most frequently asked questions with regards to the COVID-19 vaccine.
The ministry said that the most commonly reported side effects of the vaccine were as follows: “tiredness, headache, fever, trembling, muscle/joint pain, vomiting, diarrhea, and sore/rash/swelling where the injection was given.”
People who have already caught and overcome the virus will not be vaccinated as the existing antibodies will protect the body.
“It takes a couple of weeks on average for the vaccine to develop enough antibodies that will fight off the virus, but the same level of protection may not develop in everyone,” the ministry said.
Although the person might be protected with vaccination to a certain extent, they might still transmit the disease, said the ministry, therefore urging everyone to keep abiding by the rules of wearing a mask, paying attention to social distance and hygiene rules.
“Until the ministry announces that measures are no longer needed, people need to comply with them,” it said.
Another question citizens directed at the ministry was if other vaccines, such as smallpox and tuberculosis vaccines, would protect the person against the COVID-19. The ministry said that there is no scientific data suggesting these other vaccines would develop antibodies for the COVID-19.
If a person had been already vaccinated for influenza or pneumonitis, they need to wait at least two weeks to get the “inactivated” COVID-19 shot, the ministry said.
Turkey plans to purchase AstraZeneca vaccine through Russia
Turkey plans to purchase the COVID-19 vaccine developed by AstraZeneca and Oxford University through a Russian firm, journalist Fatih Altaylı said on Jan. 20, basing this information on a conversation with Professor Recep Öztürk, a member of the Health Ministry’s Coronavirus Scientific Advisory Board.
 Altaylı wrote in his column in Habertürk that the British-Swedish multinational pharmaceutical company AstraZeneca had authorized an anonymous Russian firm to sell and market the vaccine in the geography that Turkey is in.
Altaylı wrote in his column in Habertürk that the British-Swedish multinational pharmaceutical company AstraZeneca had authorized an anonymous Russian firm to sell and market the vaccine in the geography that Turkey is in.
Turkish authorities are said to be in contact with this Russian firm, with the hope that the two sides will strike a deal soon, Altaylı wrote.
Turkey made its first COVID-19 vaccine deal with Sinovac Biotech Ltd., saying it found the Chinese vaccine to be the safest as it’s manufactured using conventional methods.
The country already received the first batch of 3 million doses of the vaccine on Dec. 30. These 3 million doses will be administered to three million people, and they will receive the second dose from the second batch of the vaccine in 28 days, Altaylı wrote.
By the end of February, at least three million people will have received both of the doses, whereas another seven million people will have received the first dose, according to Altaylı.
Turkey also struck a deal with German company BioNTech, planning to receive 1 million doses by February and receive another 4 million doses by the end of April, Altaylı wrote.
However, as BioNTech does not provide a “risk guarantee,” the two sides have not agreed on a “full deal,” the journalist wrote.
“No risk guarantee” means that BioNTech takes no responsibility should a “risk” arise due to the vaccine. “I have been told that BioNTech wants to protect itself from compensation cases if a problem arises in vaccinated people,” Altaylı wrote, adding there is no clear information if the company has laid down this condition for all buyer countries.

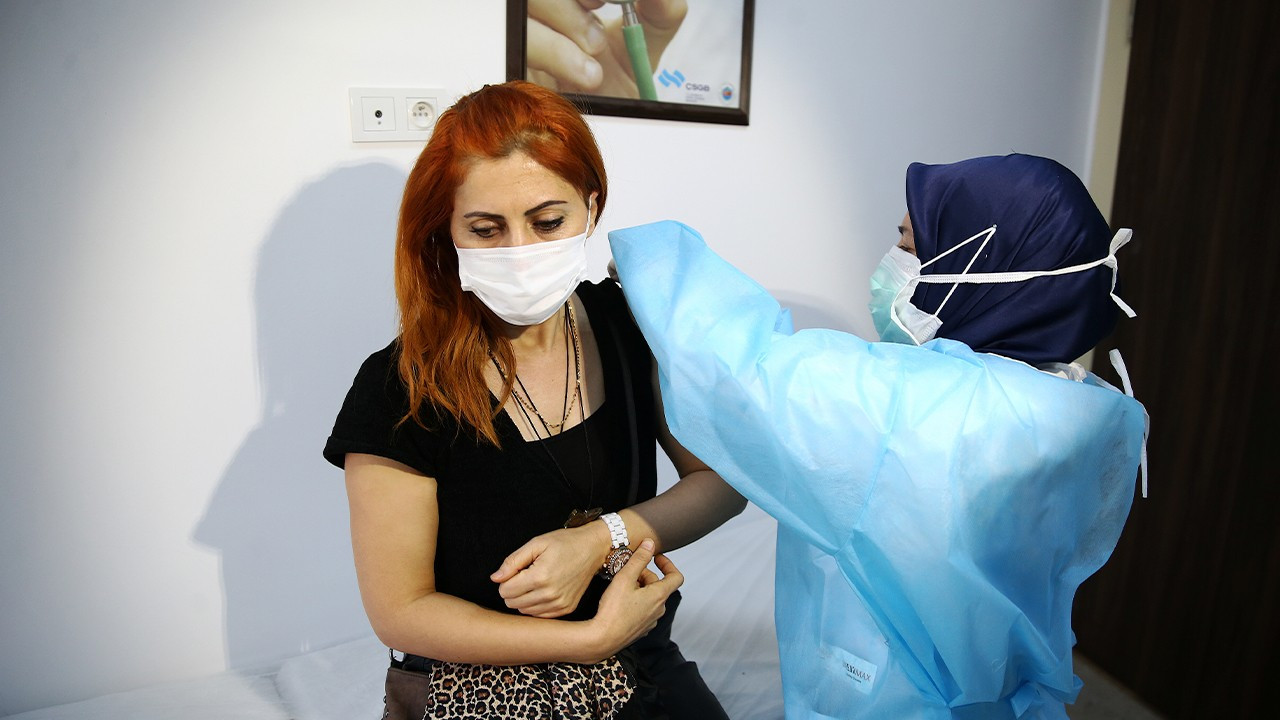 'CoronaVac will not stop infection but prevent death and heavy symptoms'Coronavirus
'CoronaVac will not stop infection but prevent death and heavy symptoms'Coronavirus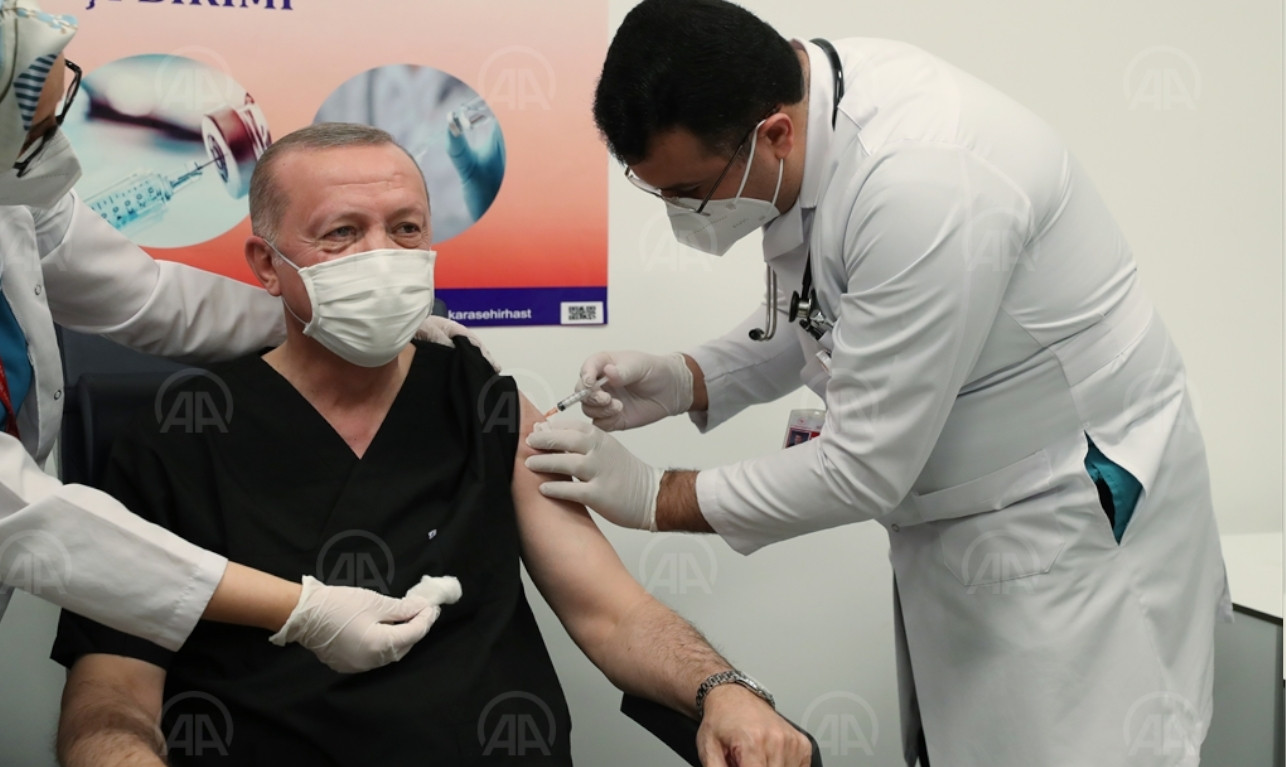 President Erdoğan receives Chinese COVID-19 vaccinePolitics
President Erdoğan receives Chinese COVID-19 vaccinePolitics